Eutectoid Microstructure
The mechanism of eutectoid transformation must transform a single solid phase into two others, both with compositions which differ from the original. Taking the eutectoid decomposition of iron as an example, austenite containing 0.8% C changes into ferrite (iron containing almost no carbon) and cementite (Fe3C, containing 25 at% carbon). Hence carbon atoms must diffuse together to form Fe3C, leaving ferrite. Nuclei of small plates of ferrite and cementite form at the grain boundaries of the austenite, and carbon diffusion takes place on a very local scale just ahead of the interface (schematic below). Thus the plates grow, consuming the austenite as they go, to form pearlite.
Notice again that this structure has a very large area of phase boundary between ferrite and cementite, so there is a surface energy penalty in forming this plate-like structure. However this is a very efficient mechanism of transformation, as C atoms on average diffuse only one plate spacing, while the interface between the new phases and the austenite traverses whole grains.
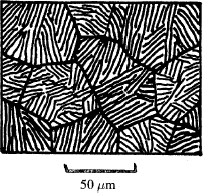
The eutectoid structure in iron has a special name: it is called pearlite (because it has a pearly look). The schematic and micrograph below show pearlite. It is important to note that pearlite is not a phase, but a mixture of two phases: ferrite and cementite.
 |
 |
