
Overview on the discovery, structure, properties and production of Carbon Nanotubes
What are Carbon Nanotubes?

Carbon is an amazingly versatile element in its ability to bond in various ways to form materials which have very different properties. The most abundant form of pure carbon on earth is graphite, which is composed of sheets of trigonally bonded carbon atoms arranged in hexagonal sheets (called graphene layers) as shown in Figure 1(a). Graphite is a soft, grey solid with high electrical conductivity along the direction of its graphene layers. Under conditions of extreme temperature and/or extreme pressure, carbon forms diamond, which is composed of tetrahedrally bonded carbon atoms as shown Figure 1(b). Diamond is a precious stone which is transparent, insulating and the hardest material known on earth. Then there also exists a whole range of closed-caged carbon structures called fullerenes, the most famous of which is the C60 molecule (Figure 1(c)) or the Buckminster fullerene which was discovered by Kroto in 1985. In 1991, Iijima, whilst studying the carbonaceous deposit from an arc discharge between graphite electrodes, found highly crystallized carbon filaments which were merely a few nanometers in diameter and few microns long. These high aspect ratio structures had a unique form – they contained carbon atoms arranged in graphene sheets which were rolled together to form a seamless cylindrical tube (Figure 1(d)), and each filament contained a ‘Russian doll’ arrangement of coaxial tubes – hence, the term ‘nanotube’ was born to describe these structures. Nanotubes can be single walled (ie. one tube) or multiwalled (ie. multiple concentric tubes).
Why are Carbon Nanotubes technologically important?
With graphene tubes parallel to the filament axis, nanotubes would inherit several important properties of ‘intra-plane’ graphite. This imparts a very unique combination of properties on this material, namely:
High aspect ratio structures with diameters in nanometers, lengths in microns
High mechanical strength (tensile strength 60GPa) and modulus (Young’s modulus 1TPa)
High electrical conductivity (10-6 ohm m typically), and for well crystallised nanotubes ballistic transport is observed
High thermal conductivity (1750-5800 W/mK)
Being covalently bonded, as electrical conductors they do not suffer from electromigration or atomic diffusion and thus can carry high current densities (107 -109 A/cm2 )
Single wall nanotubes can be metallic or semi-conducting
Chemically inert, not attacked by strong acids or alkali
Collectively, nanotubes can exhibit extremely high surface area
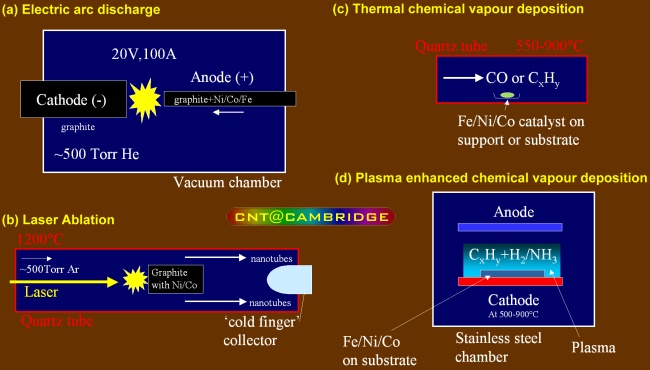
How are Carbon Nanotubes produced?
Menu
© 2003 CNT@Cambridge
web by Ken Teo